Which of the following molecules are non-polar. Covalent bond hydrogen bond ionic bond metallic bond Hydrogen bonds are the weakest because it occurs between compounds-intermolecular.
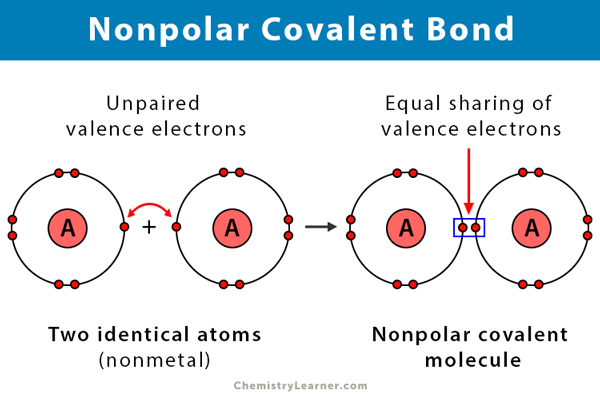
Nonpolar Covalent Bond Definition And Examples
O CC14 O CHCI3 O BH3 O H20 O CO2.
. The molecule with polar bonds will be nonp. Which of the following molecules contains a nonpolar covalent bond. Which of the following molecules contains a polar covalent bond.
The name of the HSO4- ion is sulfite. A nonpolar molecule with nonpolar bonds. Which of the following compounds contains one or more covalent bonds.
There are three sigma bonds present in hydrogen peroxide. When a covalent bond joins atoms of different elements and the bonding electrons are shared unequally the bond is. Which molecule is the only nonpolar one.
It has linear structure OCO. The following molecules contain polar covalent bonds. The other one is between two oxygen atoms where there is no unequal sharing of electrons as they have same dipole moment.
No it has a nonpolar covalent bond. In this bond which of the following best describes the charge on the carbon atom. The following molecules contain polar covalent bonds.
2Which of the following contains both ionic and covalent bonds. The other 3 occur inside the compounds-intramolecular The following molecules all contain polar bonds. Nonpolar Some examples of nonpolar molecules include noble gases carbon dioxide methane benzene homo-nuclear diatomic molecules oxygen propane butane sulfate carbon tetrachloride ethylene hydrocarbons toluene gasoline sulfur hexafluoride beryllium dichloride acetylene fats turpentine and alkanes.
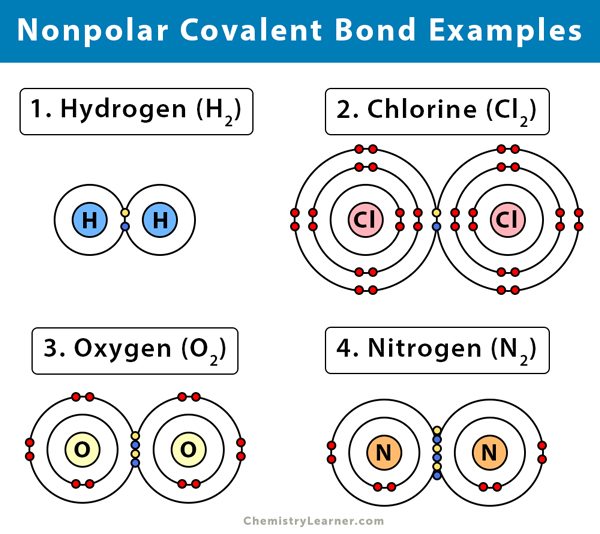
Hence only H 2 O 2 molecules have both polar and nonpolar bonds and hence the correct option is C. Does H2 have a polar covalent bond. Covalent bonds in diatomic molecules containing two atoms of.
Chemistry questions and answers. 29Which one of the following molecules is nonpolar. A polar molecule with nonpolar bonds.
No compound is completely covalent or ionic. The compound methylamine CH3NH2 contains a C-N bond. Predict the shape and bond angle for the compound carbon tetrafluoride CF4.
The geometry of the hybrid. So the correct answer is hydrogen peroxide. HCl H2O CO2 NH3 CO2 the dipole cancels out.
When a covalent bond is formed between homo atoms similar atoms like C l 2 O 2 etc. Which of the following molecules contains a nonpolar covalent bond. NaF Br2 HCl O2 MgO.
Two of them are between hydrogen and oxygen atoms which is polar in nature. State whether the following compounds contain polar covalent bonds non polar covalent bonds or ionic bonds based on their electronegativities. Then equal sharing of electrons occurs and a nonpolar.
In covalent compounds some ionic character exists. Which of the following has nonpolar bonds. Which of the following molecule contains non polar covalent bond.
Although oxygen is more electronegative. HCN is a linear molecule with 2 bond dipoles that are in the same direction and are not equal therefore the bond polarities do not cancel and the molecule is polar. A polar molecule with polar bonds.
The covalent bond length is the shorlet in which one of the following bonds. Which are overall nonpolar molecules. The resultant of the dipole.
You must draw Lewis structures and determine shape before answering 1st attempt Choose one or more. Which of the following compounds contains a polar covalent bond. A H 2 B Cl 2 C HCl D A and B Medium Solution Verified by Toppr Correct option is D.
Trigonal planar 120 degrees. So it is non polar. CO 2 is a linear molecule with 2 bond dipoles that are equal and oppositely directed therefore the bond polarities cancel and the molecule is nonpolar.

Covalent Bonds Biology For Majors I

Pin By Salma On Science Covalent Bonding Molecules Positivity

0 Comments